Vitamins
UncategorizedVitamins are organic compounds required in very small amounts by the body for growth, maintenance and sustenance of life. Funk, who in 1912 first observed that diseases such as beriberi, scurvy and pellagra can be prevented by certain components of the food. He proposed the name vitamine for these food components considering that they are all amines. Afterwards, when it was realized that all such compounds do not possess nitrogen in their structures, the name was modified to vitamin.
Classification of Vitamins:
Water-soluble vitamins, which include thiamin (vitamin B1), riboflavin (vitamin B2), niacin and nicotinamide (vitamin B3), pantothenic acid (vitamin B5), pyridoxine and related compounds (vitamin B6), cyanocobalamin and related compounds (vitamin B12), ascorbic acid (vitamin C), biotin (vitamin H) and folic acid (vitamin M).
Fat-soluble vitamins, which include retinol (vitamin A), cholecalciferol (vitamin D), tocopherols (vitamin E) and phylloquinone and related compounds (vitamin K)
STRUCTURE AND PROPERTIES OF WATER-SOLUBLE VITAMINS:
Thiamin (vitamin B1):
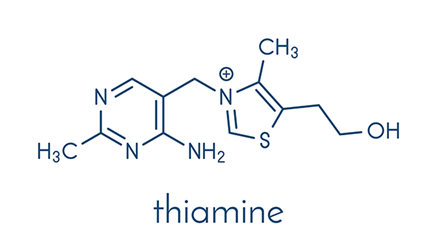
Thiamin occurs in four forms: thiamin, thiamin monophosphate (TMP), thiamin diphosphate (also known as thiamin pyrophosphate, TPP) and thiamin triphosphate (TTP):
Properties:
1) Thiamin hydrochloride is a white, needle-shaped crystalline substance.
2) It has a characteristic smell like that of yeast. In fact, the characteristic smell of yeast is due to its content of thiamin. Thiamin has a sulfurours odour and a bitter taste.
3) The compound is readily soluble in water and slightly soluble in alcohol.
4) It is stable in acid medium at room temperature but destroyed, if heated at 120o C for 30 minutes.
5) Thiamin is readily destroyed by heat in neutral or alkaline medium. It is very sensitive to alkali and can be even destroyed at room temperature in an alkaline medium.
6) The compound is converted to an inactive derivative-thiochrome by controlled oxidation (by the action of potassium ferricyanide in alkaline solution). Thiochrome has a strong fluorescence in UV rays.
7) Thiamin, when dissolved in sodium bisulphate solution at pH 5.0 cleaves into pyrimidine and thiazole.
Riboflavin (vitamin B2):
Riboflavin was isolated in a crystalline form from milk by Kuhn and co-workers in 1933. Previously it is called lactoflavin. Riboflavin has an isoalloxazine nucleus e.g., a pteridine ring with a benzene ring fused on to it. The side chain is a C5-polyhydroxy group, a derivative of ribitol, a pentahydroxy compound. Riboflavin is chemically known as 6,7-dimethyl-9-D ribitylisoalloxazine.
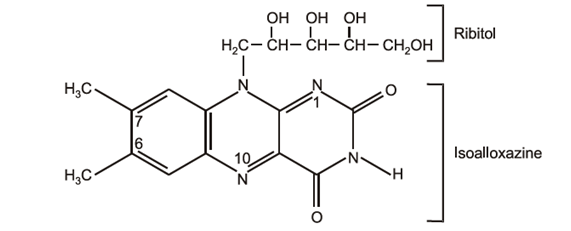
Riboflavin has two major coenzyme derivatives, namely flavin mononucleotide (FMN) which is the active component of riboflavin and is formed by the addition of a phosphate group and flavin adenine dinucleotide (FAD) which is formed by the combination of FMN with one molecule of adenosine triphosphate (ATP).
Properties
a) Riboflavin forms needle shaped orange crystals.
b) It is sparingly soluble in water and ethanol but its solubility in water is much less than thiamin.
c) Aqueous solution of vitamin B2 emits a yellow-green fluorescence.
d) Though the compound is stable to boiling in acid medium, it is readily destroyed by heat in an alkaline medium.
e) Riboflavin is sensitive to light and is destroyed if exposed to light for some time.
f) Reducing agents such as stannous chloride convert the vitamin to a colourless compound having no fluorescence.
g) When an alkaline solution of riboflavin is exposed to ultra violet rays, it is converted to a compound lumiflavin which is soluble in chloroform and has a greenish yellow fluorescence in ultra violet light.
h) When a neutral or acid solution of riboflavin is exposed to ultra violet rays, it is converted to lumichrome which has a slight blue fluorescence in ultra violet light.
Niacin (vitamin B3):
Niacin or nicotinic acid isolated it from yeast and rice polishing in order to identify the anti-beriberi vitamin in 1913. pellagra is the disease condition caused due to the deficiency of niacin in the body.
Niacin or nicotinic acid is pyridine-3-carboxylic acid. It occurs naturally in the body as its amide, niacinamide or nicotinamide. Amino group substituted into carboxylic acid forms amide group.
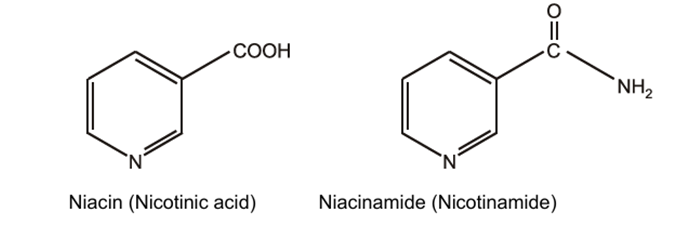
Properties
a) Niacin is a white crystalline solid.
b) While niacin is sparingly soluble in water, ethanol and glycerol, niacinamide is readily soluble in water.
c) Niacin is fairly heat stable and can withstand a temperature of 120oC for 20 minutes in acid or alkali.
d) Niacinamide is converted into niacin if heated in strong acid or alkali.
Pantothenic acid (vitamin B5):
Pure pantothenic acid was first isolated as its calcium salt from yeast by R. J. Williams in 1939. The structure of pantothenic acid consists of β-alanine and pantoic acid (dimethyl derivative of butyric acid) joined by a peptide bond. Pantothenic acid is found throughout living cells in the form of coenzyme A (CoA), a vital coenzyme in numerous chemical reactions.
Properties:
a) Pantothenic acid is a pale yellow oily liquid that can only be crystallized as its sodium, potassium or calcium salt.
b) The compound is highly soluble in water.
c) It is stable at 120oC for 30 minutes in neutral medium but is decomposed in acid or alkali solution.
d) It forms esters with alcohols.
Pyridoxine (Vitamin B6):
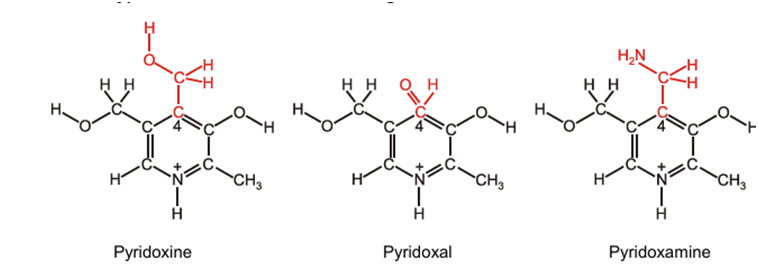
pyridoxine contains a pyridine nucleus, two primary alcoholic groups and one phenolic hydroxyl group. By replacing the –CH2OH group on position 4 of the pyridoxine molecule with –CH2NH2 and –CHO respectively, two related compounds, pyridoxamine and pyridoxal can be formed. These three compounds are interchangeable. The biologically active form or the so called coenzyme of pyridoxine is pyridoxal phosphate. This coenzyme is remarkably versatile, being involved in transaminations, decarboxylations, racemizations and numerous modifications of amino acid side chains.
Properties:
a) It forms white, odourless crystals.
b) The compound is readily soluble in water.
c) When a neutral or alkaline solution of pyridoxine is autoclaved at 120oC for 30 minutes, partial destruction of the vitamin occurs.
d) When the alkaline solution of pyridoxine is exposed to light, it is slowly destroyed.
e) Pyridoxine produces a coloured complex by reacting with 2,6-dichloroquinone chlorimide.
f) It forms salts with mineral acids and gives a violet colour with FeCl3.